Abstract
The immune suppressive properties of high-dose Cyclophosphamide are well known; however, a growing literature has described its immune stimulatory properties when applied in low dose, mediated through proliferation of effector T cells and abrogation of regulatory T cells. In order to exploit the immunogenic property of low dose Cyclophosphamide we hypothesized that its addition to Melphalan and Fludarabine in the absence of the immune suppressive effect of radiation would result in robust effector T cells proliferation through enhanced cytokine release and antigen alloreactivity. (1)
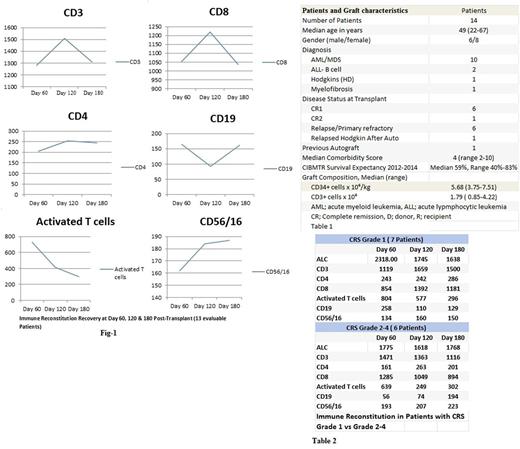
We examined the clinical outcomes and immune recovery of 14 consecutive haploidentical transplant patients with high risk hematological malignancies who received their first allogeneic transplant between 7/8/2015 and 1/10/2017. The immune recovery markers were assessed at day 60, 120 and 180 post-transplant and included absolute lymphocyte count (ALC), CD3+ CD4+, CD8+, activated T cells (CD3+HLA Dr+), CD19+ and NK cells (CD56/16+). Patients and graft characteristics are as shown in Table-1. The median duration of follow up for survivors is 15 months (range 6-25); and all but 3 patients have completed 1 year of follow up. The conditioning regimen was low dose Cyclophosphamide 14.5 mg/m2 on day -6 & day -5, Fludarabine 30 mg/m2 on days -6 to Days -2 and Melphalan 70 mg/m2 on day -3 & -2. Graft versus host disease (GVHD) prophylaxis was post-transplant Cyclophosphamide 50 mg/Kg on day +3 and day +4 along with Tacrolimus and Mycophenolate starting at day +5. Tacrolimus continued until day +180 ( Target level 5-8) and Mycophenolate continued until Day+30. Chimerism studies were performed at day +30 and +100 post-transplant by variable number tandem repeat PCR analysis of peripheral blood & bone marrow. All patients engrafted except one patient who died at day 25 prior to engraftment. Median time to neutrophil engraftment was 21 days (range 12-57). All of the evaluable 13 patients for engraftment achieved 100% donor chimerism in bone marrow (unsorted) and peripheral blood (CD3, CD33 & CD 56) by day 100. The day100 and one year overall survival were 92.8% and 85.7% respectively. Only 1/14 patients has relapsed, and the cumulative incidence of relapse is 7.74% at 1 year post- transplant. Similarly, the cumulative incidence of treatment related mortality (TRM) is 7% at 1 year post transplant. Overall 3 patients have died (2 Patients TRM and 1 Patient Relapse related mortality). TRM was related to severe CRS grade 4 in one patient at day +25 and pneumonia at day +540 in another patient. Acute GVHD grade 2-4 developed in 4 patients of whom 2 had grade 3-4. Chronic GVHD developed in 8 patients (57%) but was extensive in only 3 patients. All patients developed CRS grade as previously proposed by Lee et al.
Grade 4 CRS developed in 4/14 patients requiring ventilator support and dialysis, which led to one death during the first year post-transplant at day 25. Grade 1 CRS developed in 7 patients while 3 patients developed grade 2 CRS. Two of 4 patients with grade 4 CRS had delayed neutrophilic engraftment at day +34 and +57, and both achieved 100% donor chimerism by day 100 in bone marrow and peripheral blood. Post-Transplant immune recovery is shown in Fig-1. There was significant early recovery at day +60 in all T cell subsets. This recovery was most pronounced in activated T cells, giving support to our hypothesis. While we have observed a progressive reduction in the median number of early activated T cells at day +120 & +180, the number of helper T cells (CD4) and NK cells (CD56/16) maintained its early immune recovery. Additionally, we found more robust NK cell recovery in patients with grade 2-4 CRS (#6) compared to patients with less severe CRS grade 1 (#7). In contrast, CD4 recovery was more robust in patients with less severe CRS (Table 2)
Conclusion:
Fludarabine/Melphalan/Cyclophosphamide as a conditioning regimen prior to T-cell replete peripheral blood haploidentical transplant results in early robust immune recovery and may have the potential to decrease the risk of relapse in high risk hematological malignancies. The observed robust NK recovery in particular in patients with more severe CRS may be attributed to the lower risk of relapse. Further confirmation in a larger cohort of patients is needed to confirm these results.
(1) Madondo MT, Quinn M, Plebanski M. Low dose cyclophosphamide: mechanisms of T cell modulation. Cancer Treat Rev 2016; 42: 3-9.
Chandler: Vector Oncology: Consultancy; BMS: Consultancy; Janssen: Consultancy, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal